

3Q validation (IQ/OQ/PQ) is a core part of medical device design validation, aiming to sys...
VIEW MORE
Clamping Force: the core guarantee of closure effectiveness POM tissue closure clips need...
VIEW MORE
Vibration, impact, or compression during transportation may cause structural deformation o...
VIEW MORE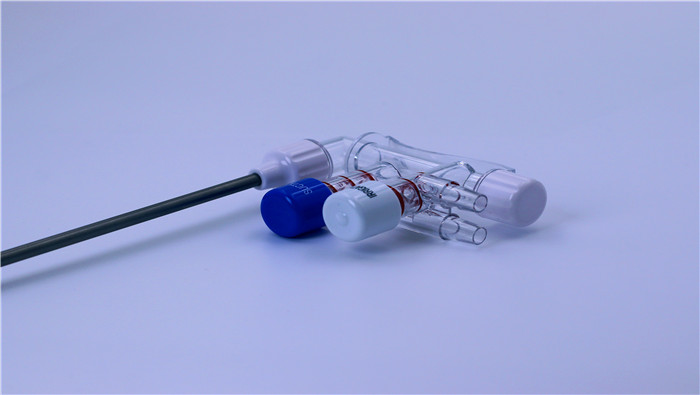
The global penetration of disposable flushing and suction devices in laparoscopic surgery ...
VIEW MORE
Traditional reusable flush suction devices need to be sterilized at high temperatures and ...
VIEW MORE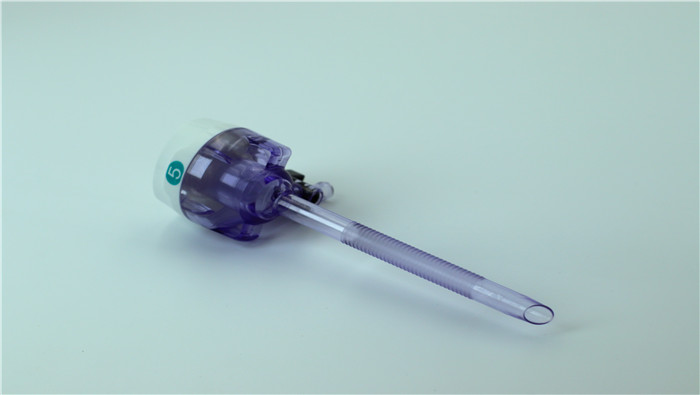
The outer diameter specification of disposable laparoscopic trocars (5mm, 10mm, 12mm) is n...
VIEW MORE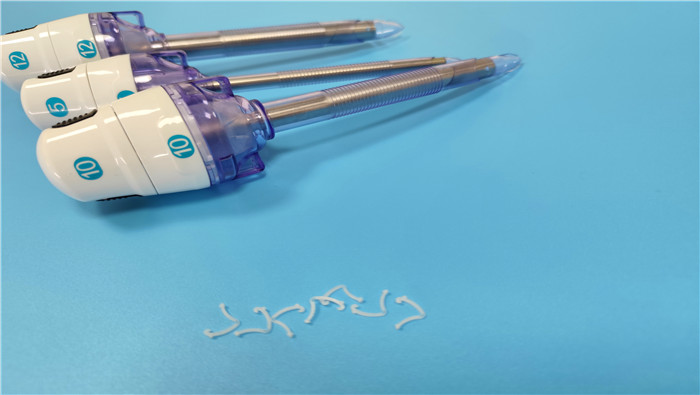
POM (polyoxymethylene) is widely used in disposable tissue closure clips (e.g., Hem-o-lock...
VIEW MORE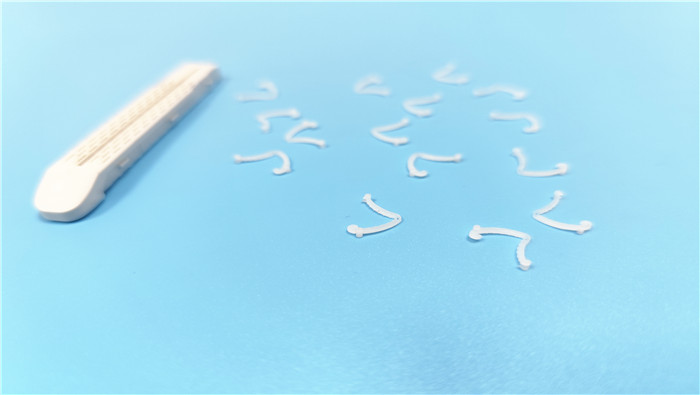
Disposable POM tissue closure clips are widely used for vascular or tissue closure in mini...
VIEW MORE
POM (polyoxymethylene) is widely used in the manufacture of medical devices such as dispos...
VIEW MORE
Sterilization is the core process to ensure the safety and effectiveness of single-use la...
VIEW MORE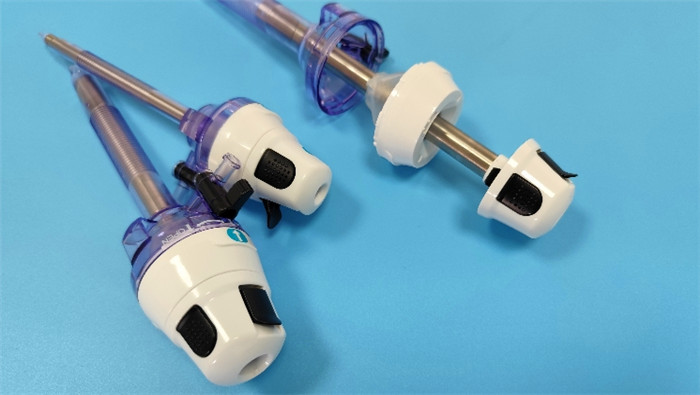
The incidence of incisional hernia after laparoscopic surgery is about 0.1%-3%, which is c...
VIEW MORE
The sealing of a single-use laparoscopic trocar (Trocar) is one of the core functions to g...
VIEW MORE+86 18361958211
marketing@cndonho.com
+86 18361958211
No.2 Zhiwei Road, Qiandeng Town, Kunshan City, Jiangsu Province, China




