
3Q validation (IQ/OQ/PQ) is a core part of medical device design validation, aiming to systematically verify the compliance and reliability of the whole process from production to performance. In the following, we analyze the key elements of 3Q verification for the characteristics of single-use laparoscopic irrigation and suction device, avoid quoting data that cannot ensure the accuracy, and optimize the presentation logic.

I. IQ (Installation Confirmation)
Goal: Verify that the production equipment, environment and documentation system meet the design requirements.
Key factors:
Equipment installation and calibration
Installation location, power and gas source configuration of key equipment (e.g., injection molding machines, assembly lines) need to comply with the requirements of technical documents.
Calibrate production-related sensors and instruments (e.g. pressure sensors, flow meters, temperature controllers) to ensure that their accuracy meets process requirements.
Confirmation of production environment
Confirm that the cleanliness, temperature and humidity control of the production environment meets the product process and regulatory requirements (e.g., to avoid contamination and risk of cross-contamination).
Environmental monitoring records need to be complete, including but not limited to particulate control, microbial load and other basic parameters.
Documentation System Audit
Confirm that equipment SOPs, maintenance records, design drawings and Bill of Materials (BOM) are complete and in controlled versions.
Review raw material supplier qualification documents (e.g. quality certificates, biocompatibility reports) to ensure supply chain compliance.
II. OQ (Operational Qualification)
Objective: To verify the ability of equipment and process to operate stably under set parameters.
Key factors:
Process Parameter Boundary Test
Verify upper and lower bounds of injection molding process parameters (e.g., temperature, pressure, time) to ensure that silicone is fully vulcanized and free of defects (e.g., air bubbles, under vulcanization).
Test batch consistency of multi-cavity mold production to ensure that critical product dimensions (e.g., cavity diameter, interface size) meet design requirements.
Functional component validation
Sealing test: Verify the sealing performance of the flush attractor through pressure hold test to ensure no leakage.
Valve function validation: Test the response speed and repeatability of the rinse/suction mode switching to confirm its reliability.
Process Control Capability
Evaluate process stability of key process parameters (e.g., analyze data distribution through statistical methods) to ensure process robustness.
Verify the effectiveness of online inspection systems (e.g., visual inspection, pressure testing) to ensure defect detection capability.
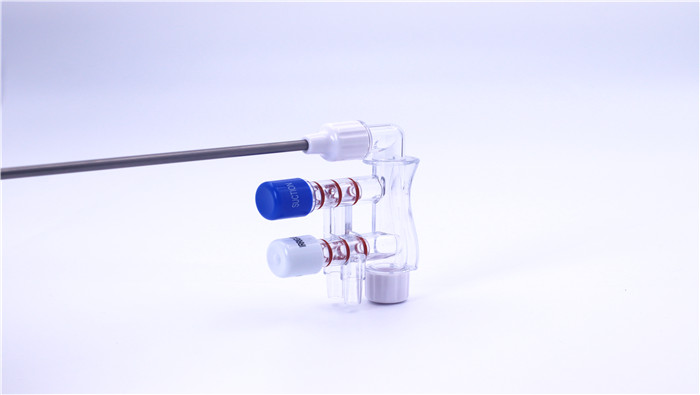
III. PQ (Performance Qualification)
Goal: Verify that the final product performance meets clinical needs and regulatory requirements.
Key factors:
Biocompatibility assessment
Complete the necessary biocompatibility testing according to international standards (e.g. ISO 10993 series), including basic items such as cytotoxicity and sensitization.
Verify chemical safety (e.g., dissolution, residues) of patient-contact components.
Sterilization and Packaging Validation
Sterilization validity: Confirm the level of sterility assurance of the sterilization process through bioindicator challenge testing.
Residue control: Ensure that sterilization residues (e.g., ethylene oxide) meet the requirements of relevant international standards.
Packaging Integrity: Verify the sealing and bacterial barrier of the packaging during the expiration date through accelerated aging test.
Clinical function simulation testing
Fluid performance verification: simulate real surgical scenarios to test the stability of flushing flow and suction negative pressure.
Fatigue test: simulate multiple use scenarios (e.g. repeated plugging and unplugging, mode switching) to verify the durability of the instruments.
Extreme condition test: Evaluate the functional reliability of the instruments in non-ideal environments (e.g., low temperature, high temperature).
Risk management and traceability system
Identify key risk points (e.g. seal failure, valve sticking) through risk analysis tools (e.g. FMEA) and develop control measures.
Establish a complete production batch traceability system to ensure the traceability of the whole chain from raw materials to finished products.

IV. Tthe integration and output of 3Q verification
Data integrity management
Ensure that all test data is traceable and reviewable, in line with the basic principles of data integrity (e.g. ALCOA).
Reporting and Review
Generate IQ/OQ/PQ validation reports, document deviation handling and corrective and preventive actions (CAPA), and approve by the quality department.
Continuous Improvement
Optimize process parameters or design details based on validation results, update technical documentation and risk management documents.
Conclusion
The 3Q validation of disposable laparoscopic irrigation and suction devices should be centered on “equipment compliance - process robustness - product reliability”, and the core is to prove the overall compliance of its design, production and performance through scientific methods. Enterprises should focus on the rigor of the validation logic and avoid relying on
+86 18361958211
marketing@cndonho.com
+86 18361958211
No.2 Zhiwei Road, Qiandeng Town, Kunshan City, Jiangsu Province, China




