
Disposable POM tissue closure clips are widely used for vascular or tissue closure in minimally invasive surgery due to their high rigidity, abrasion resistance, and ease of operation. However, the following risks and potential failure modes need to be alerted for its clinical use:
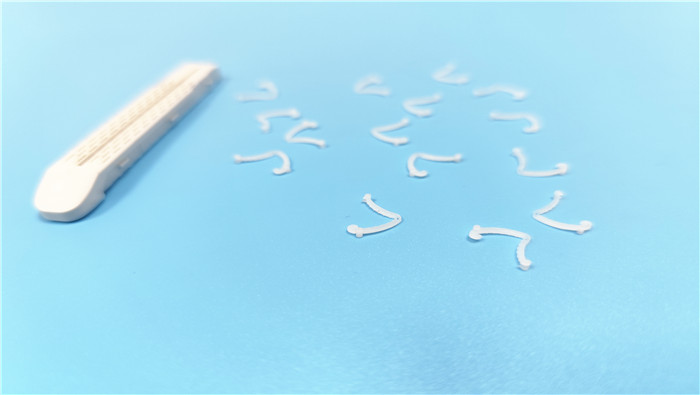
I. Intraoperative Risk Points
Insufficient Clamp Closure
Risk manifestation: Failure of the clamp body to completely close the blood vessel or tissue, resulting in postoperative blood seepage or slippage of the clamp.
Causes:
Angular deviation of the operator, with the clamp arm not acting perpendicularly to the tissue;
Inappropriate choice of clamp size (e.g., mismatch between the length of the clamp body and the thickness of the tissue).
Failure of the locking mechanism
Risk: Failure of closure due to incomplete engagement of the locking mechanism or unintentional unlocking of the locking mechanism during surgery.
Causes:
excessive tolerance in the design of the clasp (clearance > 0.05 mm);
deformation of the clasp due to uneven force of the intraoperative clamping device (e.g. forceps).
Excessive tissue compression
Risk: The clamp exerts excessive pressure on the tissue, resulting in local ischemia or necrosis.
Causes:
excessive clamping stroke (e.g., design defects);
repeated clamping in the same position resulting in secondary injury.

II.Long-term postoperative risks
Dislocation or dislodgement of the clip
Risk: The clip is dislodged from its original position, leading to delayed hemorrhage or organ damage.
Causes:
Insufficient coefficient of friction between the clip body and the tissue contact surface (defective design of the anti-slip teeth);
Reduced clamping force due to postoperative tissue oedema.
Material biocompatibility problems
Risk: Localized inflammatory reaction or granuloma formation due to foreign body encapsulation.
Causes:
migration of residual monomers or additives from the POM material;
immunological reaction due to oxidation products on the surface of the material after sterilization.
Image interference
Risk: Artifacts on CT/MRI, which may affect the postoperative assessment.
Cause analysis:
The composition of the sandwich containing the developer is incompatible with the imaging technology.
III.Failure Analysis
Material Failure
Fatigue Fracture: Repeated opening and closing or prolonged stress in the body leads to molecular chain breakage and cracks in the body.
Environmental degradation: POM hydrolysis triggered by long-term immersion in body fluids (especially products with insufficient autoclave pretreatment).
Structural design defects
Concentration of stress in the latch: the rounded corner of the root of the latch is too small (R<0.1mm), resulting in local stress exceeding the yield strength of the material.
Clamp arm rigidity imbalance: one side of the clamp arm rigidity is too high, resulting in asymmetric deformation when closed.
Insufficient process control
Injection molding defects: shrinkage, bubbles, etc. to reduce the effective cross-sectional area of the clamp body, the local strength decreased by more than 50%.
Dimensional overshoot: the length or thickness deviation of the clamp body>±0.1mm, affecting the consistency of the clamping force.
Sterilization compatibility problems
Ethylene oxide residue: insufficient resolution leads to material surface embrittlement, and the impact resistance of the clamp body decreases.
Radiation degradation: Gamma radiation triggered POM molecular chain breakage, clip closure force attenuation rate > 10%.

IV.Risk prevention and control and improvement strategy
Design optimization
Increase the density and depth of anti-slip teeth (tooth height ≥ 0.2mm), to improve the clamping stability;
Finite Element Analysis (FEA) is used to optimize the stress distribution of the locking structure to avoid local overload.
Process Enhancement
Mold temperature control precision ±1℃ during the injection stage to ensure uniform crystallinity (β crystalline type ≥60%);
Introducing a visual inspection system for 100% full inspection of latch engagement status and surface defects.
Clinical Practice
Pre-operative clip-tissue matching assessment (e.g. ultrasound measurement of blood vessel diameter);
Avoid direct contact between clips and energy instruments (e.g. electrosurgical knives) to prevent thermal damage.
Sterilization and quality control
Use low-temperature plasma sterilization instead of gamma radiation to reduce material degradation;
Establish a comparative database of clip closure force before and after sterilization, and set a threshold for attenuation rate (≤5%).
Conclusion
The clinical risks and failure modes of POM tissue closure clips need to be controlled through the whole chain of “design-process-operation”. Future R&D should focus on material modification (e.g., glass fiber reinforced POM), intelligent clamping force feedback system, and degradable alternatives, so as to fundamentally improve product reliability and upgrade the safety of minimally invasive surgery.
+86 18361958211
marketing@cndonho.com
+86 18361958211
No.2 Zhiwei Road, Qiandeng Town, Kunshan City, Jiangsu Province, China




